Achievements
 Clinical Trials
Clinical Trials
- Extensive experience including all therapeutic areas with the majority of clinical trials being in Oncology, CNS, Respiratory, Cardiology, Diabetes and Hepatology.
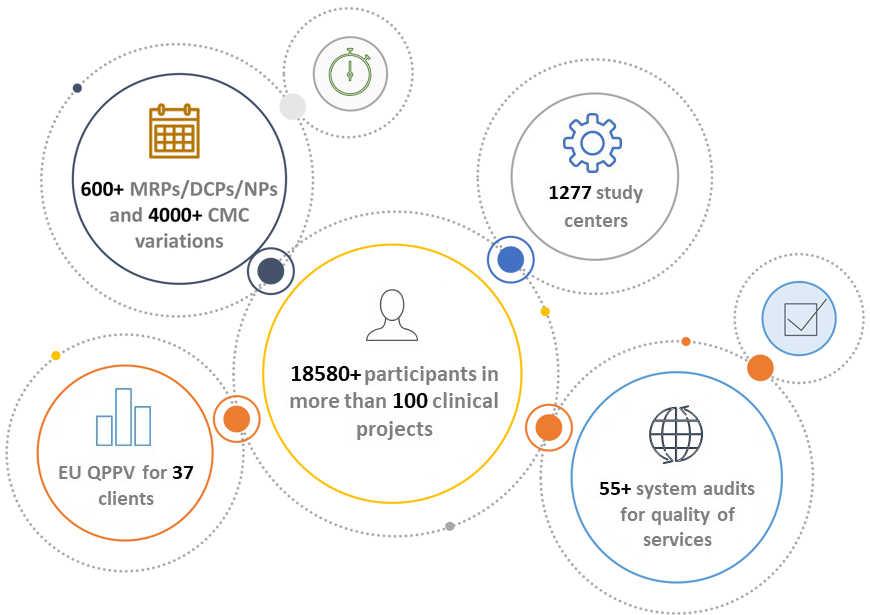
- More than 83 trials of several designs/phases (Interventional/Observational, PhII-PhIV, multi-center, national/multinational) with more than 16.000 Patients and more than 1180 Study Centers.
- Certified by the French Ministry of Higher Education and Research as an approved organisation for carrying out R&D activities for the account of private companies.
- Active participation in R&D projects of basic research with the aim to develop and invest in innovative new health technology.
- Continuous collaboration with over 160 Key Opinion Leaders.
- Management of thousands of study centers.
Pharmacovigilance
- Provision of EU QPPV for 35 pharma companies.
- Assessment of PV Quality System via 30 external Audits by international pharma companies/Contract Safety Surveillance Organisations (CSO) with successful outcomes (no critical findings).
- Own validated safety database.
- More than 10 years of experience.
- Over 50 active clients.
Regulatory Affairs
- Established collaborations with multinational pharma companies.
- Hundreds diligence dossier reviews, including gap analyses of Quality and CMC documentation.
- For more than 10 multinational pharma companies, Pharmassist functions as their sole RA representative, providing full regulatory support.
- Hundreds of due diligence dossier reviews, including gap analyses of Quality and CMC documentation.
- Hundreds of MRPs/DCPs/NPs.
- Thousands of CMC variations.
- Trusted partner during several major multinational pharma mergers and acquisitions for Marketing Authorisation transfers and other regulatory activities.
- Established network of experts in 33 EU Countries.
- More than 18 years of experience.
Medical Affairs
- Preparation of Briefing Documents in the context of seeking Scientific Advice from EMA and other EU Regulatory Authorities.
- Successful preparation of CTD Module 2, 4 and 5 documents.
- Organization and conduct of Scientific Advisory Boards.
- Preparation and publication of scientific articles.
- Extensive experience in medical evaluation of PV data.
- Preparation of study documents for several clinical studies.
Quality Management
- Certified since 2011, according to ISO 9001 standards.
- Frequently audited by major Multinational Pharmaceutical Companies for quality of services.
- Successfully assessed through 2 Ethical Assessment/Workplace Conditions Assessment (WCA).
 Clinical Trials
Clinical Trials